Comparing the SN1 and SN2 Reactions
In nucleophilic substitution reactions, a bond between carbon and a leaving group (C–LG) is broken, and a new bond between carbon and a nucleophile (C–Nu) is formed.
Nucleophilic substitution reactions of alkyl halides occur through two main pathways. The key difference lies in the timing of the bond-forming and bond-breaking steps.
- The SN1 mechanism (Substitution, Nucleophilic, UNImolecular rate determining step) generally passes through two steps; first, a (slow, rate-determining) breaking of the C–LG bond on the substrate to form an intermediate carbocation, followed by (fast) addition of a nucleophile to the carbocation (form C–Nu) to give the substitution product (there is often a third acid-base step which follows the substitution reaction when neutral nucleophiles like H2O or ROH are used)
- The SN2 mechanism (Substitution, Nucleophilic, Bimolecular rate determining step) occurs in a single, concerted step: attack of the nucleophile on the backside of the C–LG bond, passing through a transient five-membered transition state en route to a tetrahedral product where configuration at the carbon has been inverted.
Since the rate-determining step in the SN1 is formation of a carbocation, it can be helpful to think of the “big barrier” to the SN1 reaction as being carbocation stability, That is, any factor which leads to the increased stability of a carbocation intermediate will increase the rate of the SN1. That’s why this pathway tends to be favored by tertiary alkyl halides, since the order of carbocation stability generally proceeds tertiary > secondary > primary (See article: Carbocation Stability)
Similarly, since the rate-determining step of the SN2 is the backside attack of a nucleophile on carbon, it can be helpful to think of the “big barrier” to the SN2 reaction as being steric hindrance (See article: Steric Hindrance). Any factor which increases the difficulty with which the nucleophile can access the sigma* orbital of the C–LG bond will result in a slower reaction, which helps us to rationalize why the SN2 is faster with methyl and primary alkyl halides than for secondary and tertiary alkyl halides.
Owing to these two different mechanisms, the SN1 and SN2 reactions exhibit key differences in
- observed rate laws (unimolecular for SN1, bimolecular for SN2)
- patterns of stereochemistry (retention + inversion for SN1, inversion for SN2)
- relative rates of reaction for primary, secondary, and tertiary alkyl halides (tertiary > secondary > primary for SN1, primary > secondary > tertiary for SN2
Additionally, the SN1 and SN2 reactions are sensitive to the identity of the solvent and of the strength of the participating nucleophile. More detail below!

Table of Contents
- But First: The Story Of The Cats And The Comfy Chair
- The SN1 Proceeds Through A Stepwise Mechanism. The SN2 Proceeds Through a Concerted Mechanism
- Reaction Coordinate Diagrams of the SN1 and SN2 Reactions
- The Rate Laws of the SN1 and SN2 Reactions
- Primary, Secondary, and Tertiary Alkyl Halides in SN1 and SN2 Reactions
- Comparing the Stereochemistry of SN1 and SN2 Reactions
- Solvents and Nucleophiles – SN1 Reaction
- Solvents and Nucleophiles – SN2 Reaction
- Back To The Cats
- Notes
- (Advanced) References and Further Reading
1. But First: The Cats And The Comfy Chair
But first – have you ever heard the story of the cats and the comfy chair?

Cat #1 finds Cat #2 on hiscomfy chair and wants to sit. He hastwo options.
- He can wait for Cat #2 to leave, and then sit in the comfy chair.
- He can kick the Cat #2 out of his comfy chair.
Think about that for a second. In the meantime, let’s compare the SN1 and the SN2.
2. The SN1 Proceeds Through A Stepwise Mechanism. The SN2 Proceeds Through a Concerted Mechanism
Let’s compare the mechanisms of the SN1 and SN2 reactions first, since every other difference we will observe is a consequence of their different mechanisms. (Generally, proposing a mechanism only comes after collecting a lot of experimental evidence (e.g. rate laws, stereochemistry, relative rates, etc.) , but since this isn’t an Agatha Christie novel, we’re giving away the ending first).
The SN1 generally passes through a two-step “stepwise” mechanism where
- The leaving group leaves (break C–LG) to give a carbocation intermediate (slow, rate-determining step)
- The resulting carbocation intermediate is attacked by a nucleophile to give a new product (form C–Nu) (fast step)
(In many cases, there is often a third step involving deprotonation of the nucleophile to give a neutral product, especially if the nucleophile is neutral)

Note that the carbocation intermediate has a trigonal planar geometry at carbon, and its empty p-orbital can undergo addition by a nucleophile equally well at either face. In cases where substitution occurs on a stereogenic carbon (aka “chiral carbon”) this can result in a mixture of retention and inversion of stereochemistry, relative to the configuration of the original alkyl halide. [Note 1]
In contrast, the SN2 reaction passes through a one-step concerted mechanism where one equivalent of nucleophile adds to one equivalent of substrate, resulting in formation of a new bond to the nucleophile and the loss of a leaving group from carbon.
This is achieved through donation of a pair of electrons from the nucleophile into the empty, antibonding sigma-star (σ*) orbital on the backside of the C–LG bond. The carbon-nucleophile bond (C-Nu) forms at the same time that the carbon-leaving group bond (C-LG) breaks.
Since carbon cannot comfortably accommodate more than four bonding partners at one time these two bonds are considered to have partial bonding character in the transition state (note the dashed lines, below) where carbon adopts a “trigonal bipyramidal” geometry.
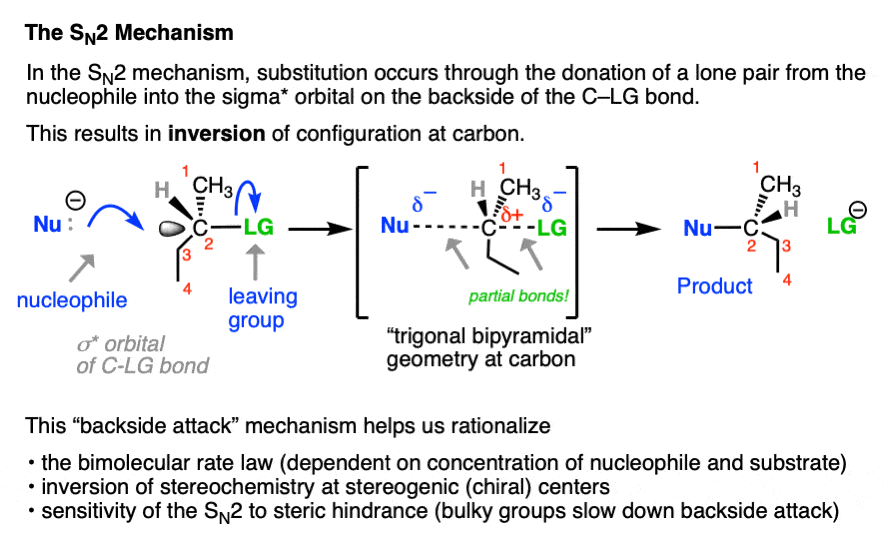
As the C-Nu bond forms and the C-LG bond breaks, the three “trigonal” substituents on the “equator” of the carbon relax to a tetrahedral geometry, with the difference that they are completely inverted from their original position (like the often-invoked metaphor of the “umbrella turning inside-out in a strong wind”) .
3. Reaction Coordinate Diagrams Of The SN1 and SN2 Reactions
One way to visualize the differences between these two mechanisms is to sketch out their reaction coordinate diagrams, where we graph changes in potential energy (vertical axis) the starting materials pass along the “reaction coordinate” toward their conversion into products (horizontal axis) (These diagrams resemble a graph of changes in altitude (also potential energy!) experienced by a hiker navigating a mountain pass between two destinations.)
In these diagrams the “peaks” (local maxima) represent transition states whereas “valleys” (local minima) represent intermediates. (A transition state is a transient species with partial bonds. An intermediate is a potentially isolable species. )
The reaction coordinate diagram of the SN1 reaction has a two peaks, representing the two transition states (Step 1 and Step 2, respectively) flanking a single “valley” representing the carbocation intermediate.
Each step of the process has anactivation energyrepresented by the difference in energy between the reactant and the transition state.
The rate-determining step of a reaction is the step requiring the highest activation energy, that is, the largest change in potential energy from reactant to transition state. In the SN1 reaction, the rate determining step is (illustrated in pink) loss of the leaving group from the alkyl halide to give the carbocation.

The reaction coordinate diagram of the SN2 reaction shows only a single transition state (one “peak”) corresponding to the concerted formation of C-Nu and breakage of C-LG without any intermediate. (Note 2)
One note – the difference in energy between the starting material and the product reflects the fact that the leaving group is generally a weaker base than the nucleophile, as well as the difference in bond strengths to carbon. (See article: What Makes A Good Leaving Group)

4. The Rate Laws Of The SN1 and SN2 Reactions
One way of probing the mechanism of a given substitution reaction is to measure the changes in reaction rates when the concentration of both nucleophile and substrate are varied.
Since the rate-determining step of the SN1 reaction is loss of a leaving group from the substrate – a unimolecular reaction – the rate of product formation in the SN1 should depend only on the concentration of substrate.
Rate =k [Concentration of alkyl halide]
Doubling, tripling, or quadrupling the concentration of substrate should thus result in a doubling, tripling, or quadrupling of the rate of product formation, respectively.
Doubling, tripling, or quadrupling the concentration of nucleophile on the other hand should have no effect on the reaction rate since the nucleophile is not involved in the rate-determining step.

The SN2 reaction, by contrast, has a bimolecularrate-determining step where one equivalent of nucleophile combines with one equivalent of substrate.
The overall rate law of the SN2 is thus dependent on both the concentration of substrate and the concentration of nucleophile, taking the form:
Rate = k [Concentration of alkyl halide] [Concentration of nucleophile]
We say this is “first-order” in nucleophile and “first-order” in substrate, or “second order” overall.
- Doubling the concentration of substrate will double the rate of formation of product.
- Likewise, doubling the concentration of nucleophile will also double the rate of product formation.
- Doubling the concentration of substrate and nucleophile will result in a quadrupling of the rate of product formation.

5. The Effect of Substrate On The Rate of SN1 and SN2 Reactions
The dependence of the rate of the SN1 on carbocation stabilityand the rate of the SN2 on steric hindrance means that the trends of their reaction rates with primary, secondary, and tertiary alkyl halides proceeds in opposite directions.
The rate-determining step of the SN1 reaction is formation of a carbocation. Since tertiary carbocations are more stable than secondary carbocations which are in turn far more stable than primary (and methyl) carbocations, we should observe that the rate of SN1 reactions is fastest with tertiary alkyl halides.
This is indeed the case, as illustrated when various alkyl halides are subjected to typical SN1 conditions (a poorly nucleophilic, polar protic solvent such as H2O) [Ref]

(Note that these are relative rates, where the rate of substitution at t-butyl bromide 1.2 × 106 is measured relative to the rate of substitution at ethyl bromide (1))
The rate determining step of the SN2 reaction is backside attack of a nucleophile on an alkyl halide. Since hydrogen atoms are smaller than carbon atoms, we should expect that the rate of SN2 reactions is fastest with methyl and primary alkyl halides and slowest with tertiary alkyl halides.
This also agrees with experiment, as shown below when a variety of alkyl halides are treated with the strong nucleophile NaCN.

6. Comparing The Stereochemistry of SN1 and SN2 Reactions
Thestereochemistryof the products relative to those of the starting material are also a useful probe of SN1 versus SN2 pathway.
When a stereogenic center loses a leaving group to become a trigonal planar carbocation, it loses chirality.
Since the resulting carbocation can be attacked on either face by a nucleophile, the resulting product will be a mixture of retention and inversion of stereochemistry.

An SN2 reaction that occurs on a stereogenic carbon will result ininversionof configuration, but willretain optical purity.

Note that inversion still happens whether or not the starting material is chiral or not; it just won’t be observable.
7. Solvents And Nucleophiles – SN1
The SN1 reaction tends to occur when alkyl halides capable of forming reasonably stable carbocations are dissolved in polar protic solvents that are capable of acting as nucleophiles.
Loss of a leaving group to give a carbocation results in the formation of a transient ion pair (i.e. the carbocation and the leaving group) from a neutral species (i.e. the alkyl halide)
Just as salts like NaCl are unlikely to dissolve in non-polar solvents like hexane (dielectric constant, ε = 2), carbocation formation is much more favorable in polar solvents like water (ε = 78), alcohols (ε ~ 20-40), or carboxylic acids (e.g. formic acid, ε = 51) , which are able to stabilize charges through hydrogen bonding or other dipolar interactions.
Carbocations resemble neutral compounds of boron (e.g. BF3), in that they are excellent Lewis acids containing 6 valence electrons and an empty p-orbital. Once formed, carbocations readily undergo addition even with poor Lewis bases to give products with a full octet around carbon.
So in practice, this generally means the solvent is the nucleophile, since it is present in much higher concentrations relative to anything else. [Note 3]

8. Solvents and Nucleophiles – SN2
SN2 reactions can certainly be carried out in polar protic solvents like alcohols, especially with primary alkyl halides.
But since the rate of the SN2 isn’t dependent on carbocation formation, a much wider variety of (less polar) solvents may be used.
Furthermore, alkyl halides are poorer electrophiles than carbocations. This is actually a good thing, since they are much less likely to undergo side reactions like rearrangement or elimination, or react with the first Lewis base they see.
For practical purposes, SN2 reactions tend to be carried out with stronger (i.e. charged) nucleophiles. A wide variety of nucleophilic partners can be used, which makes the SN2 extremely versatile, especially with primary alkyl halides. (See article – Why The SN2 Reaction Is Powerful)

Polar aprotic solvents such as DMSO, acetone, DMF and acetonitrile are often chosen for SN2 reactions since they are polar enough to dissolve the reaction partners, but cannot form hydrogen bonds to the nucleophile. This has the practical effect of making the nucleophile less bulky, since the nucleophile isn’t surrounded by a shell of solvent molecules everywhere it goes.
9. Back To The Cats
So does the story about the cats and the comfy chair make more sense now?
- In the SN2, the nucleophile (Cat #1) forms a bond to the substrate (comfychair) at the same time the leaving group (Cat #2) leaves.
- In the SN1, the leaving group (Cat #2) leaves the substrate (comfy chair), and then the nucleophile (Cat #1) forms a bond.
If this makes sense, you might be ready for the Quick N’ Dirty Guide to SN1/SN2/E1/E2 reactions. Otherwise, join us for our next post when we discussrearrangement reactions.
Don’t forget – you can download a free 1-page Summary Sheet of SN1 vs SN2 reactions containing all the material on this blog post here:Download SN1 vs SN2 Summary Sheet PDF
Notes
Related Articles
- The SN1 Mechanism
- The SN2 Mechanism
- Identifying Where Substitution and Elimination Reactions Happen
- Polar Protic? Polar Aprotic? Nonpolar? All About Solvents
- Steric Hindrance is Like a Fat Goalie
- What makes a good leaving group?
- Primary, Secondary, Tertiary, Quaternary In Organic Chemistry
- Deciding SN1/SN2/E1/E2 (1) – The Substrate
- Introduction to Rearrangement Reactions
Cat Illustration by my talented cousin, political cartoonist Graeme MacKay
Note 1.[Intimate ion pairs can affect this].
Note 2.[If the nucleophile is neutral, e.g. H2O, then there will be a second, lower-energy transition state corresponding to the deprotonation step]
Note 3. [It is possible to trap carbocations with external nucleophiles.]
UPDATE . The most perfect cat video ever. Thanks to Alex Roche (Rutgers U.) for sending.
Quiz Yourself
[quizzes]
(Advanced) References and Further Reading
Solvolytic Displacement Reactions by Andrew Streitweiser is an oldie but a goodie in terms of compiling a lot of information (particularly reaction rates) of SN1 and SN2 reactions. Available on the Internet Archive for 1-hour loans here.
- Reaction kinetics and the Walden inversion. Part VI. Relation of steric orientation to mechanism in substitutions involving halogen atoms and simple or substituted hydroxyl groups
W. A. Cowdrey, E. D. Hughes, C. K. Ingold, S. Masterman, and A. D. Scott
J. Chem. Soc. 1937, 1252-1271
DOI: 10.1039/JR9370001252
The points listed in the summary are worth reading for understanding what influences the SN1 and SN2 pathways. - Mechanism of substitution at a saturated carbon atom. Part XXVI. The rôle of steric hindrance. (Section A) introductory remarks, and a kinetic study of the reactions of methyl, ethyl, n-propyl, isobutyl, and neopentyl bromides with sodium ethoxide in dry ethyl alcohol
I. Dostrovsky and E. D. Hughes
J. Chem. Soc. 1946, 157-161
DOI: 10.1039/JR9460000157
Table I in this paper shows the reduction in reaction rate for the SN2 reaction of R-Br with OEt- when R goes from methyl -> ethyl -> n-propyl -> isobutyl -> t-amyl. This can be attributed to steric hindrance, as backside attack of the substituted carbon becomes increasingly challenging. - Mechanism of substitution at a saturated carbon atom. Part III. Kinetics of the degradations of sulphonium compounds
John L. Gleave, Edward D. Hughes and Christopher K. Ingold
J. Chem. Soc. 1935, 234-244
DOI: 10.1039/JR9350000236
This is a useful paper – in the beginning the terms “SN1” and “SN2” are introduced and defined, and Figs. 1 and 2 depict how the two mechanisms can compete depending on the structure of the substrate. - Influence of poles and polar linkings on the course pursued by elimination reactions. Part XVI. Mechanism of the thermal decomposition of quaternary ammonium compounds
E. D. Hughes, C. K. Ingold, and C. S. Patel
J. Chem. Soc. 1933, 526-530
DOI: 10.1039/JR9330000526
At the end of this paper, the authors make an important point: “When the various series can be more fully filled in, what has been described as a “ point ” of mechanistic change will probably appear as a region, and thus, just as with reaction (A), we now generalise the original conception of reaction (B) by the contemplation of a range of mechanisms, (Bl)-(B2), both extremes of which have been experimentally exemplified”. Basically, the SN1 and SN2 mechanisms as taught are two extremes of a continuum, and in practice most reactions lie somewhere in between. - Mechanism of substitution at a saturated carbon atom. Part IX. The rôle of the solvent in the first-order hydrolysis of alkyl halides
Leslie C. Bateman and Edward D. Hughes
J. Chem. Soc. 1937, 1187-1192
DOI: 10.1039/JR9370001187 - The Common Basis of Intramolecular Rearrangements. VI.1 Reactions of Neopentyl Iodide
Frank C. Whitmore, E. L. Wittle, and A. H. Popkin
Journal of the American Chemical Society 1939, 61 (6), 1586-1590
DOI: 1021/ja01875a073
An early paper demonstrating that SN1 reactions can be induced by reaction of an alkyl halide with silver salts. In this case, the neopentyl cation quickly rearranges to the significantly more stable t-amyl cation, and those products are obtained. - Reaction kinetics and the Walden inversion. Part I. hom*ogeneous hydrolysis and alcoholysis of β-n-octyl halides
Edward D. Hughes, Christopher K. Ingold and Standish Masterman
J. Chem. Soc. 1937, 1196-1201
DOI: 10.1039/JR9370001196 - Reaction kinetics and the Walden inversion. Part IV. Action of silver salts in hydroxylic solvents on β-n-octyl bromide and α-phenylethyl chloride
Edward D. Hughes, Christopher K. Ingold and Standish Masterman
J. Chem. Soc., 1937, 1236-1243
DOI: 10.1039/JR9370001236
These two papers examine reactions of 2-octyl halides in an attempt to see if pure SN1 or SN2 pathways on the same substrate can be favored simply by varying the reaction conditions.