What is the normality of NaCl?
Sodium chloride has a valence of 1 and a molecular weight of 58.443. Therefore, the equivalent weight is 58.443/1 or 58.443. 1 gram of NaCl is dissolved into 0.05 L of water, so the normality of the solution is 1/(58.443 x 0.05) or 0.342.
For example, if you wanted a 0.5 M solution, you would use 0.5 x 58.44 g/mol of NaCl in 1 L of solution or 29.22 g of NaCl.
0.9% NaCl solution has a molarity of 154 mmol/L whether the solution volume is 1 dL, 1 L, 1 μL, or an Olympic-size swimming pool!
0.01 M NaCl means 0.01 moles of NaCl in a total volume of 1 liter. Converting this to amount needed for 250 ml (0.025 L), you get... 0.01 mole/L x 0.25 L = 0.0025 moles of NaCl required.
To make a 1 N sodium chloride solution:
The molecular weight of NaCl is 58.5. Gram equivalent weight of NaCl = molecular weight/1 (valency). So dissolve 58.5 grams of NaCl in distilled water and make up to one liter.
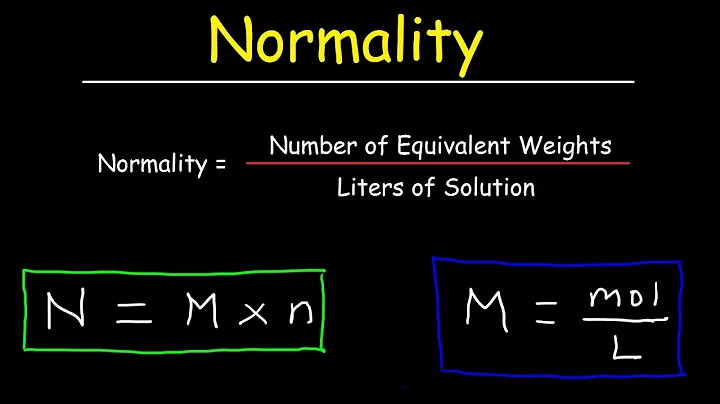
- We already know that:
- Normality = No of gram equivalent in a solute / Volume of solution (in L), and.
- Molarity (or M) = The number of moles of solute / Volume of solution in L.
- Now, N/M = No of gram equivalent / Number of moles of solute…(
To make a 0.1M NaCl solution, you could weigh 5.844g of NaCl and dissolve it in 1 litre of water; OR 0.5844g of NaCl in 100mL of water (see animation below); OR make a 1:10 dilution of a 1M sample.
Hypotonic solution with 0.5 percent NaCl causes RBC to expand and rupture owing to the difference in osmotic pressure. The concentration of solutes in a hypotonic solution is lower than in another solution.
A solution containing 0.5% salt is hypotonic with respect to the cell.
Therefore, 0.9% saline is considered to be an isotonic solution, means in the range of 275–295 mOsm/kg of water (osmolarity and osmolality of saline solution are equal). The osmotic coefficient must be used to convert concentration to activity and to describe why 0.9% saline is isotonic.
Is normal saline 0.09% NaCl?
Sodium Chloride 0.9% intravenous infusion is an isotonic solution, with an approximate osmolarity of 308 mOsm/l. The pharmacodynamic properties of the solution are those of the sodium and chloride ions in maintaining the fluid and electrolyte balance.
A 1 molar (M) solution will contain 1.0 GMW of a substance dissolved in water to make 1 liter of final solution. Hence, a 1M solution of NaCl contains 58.44 g.
Wt. of NaCl (g) = 0.025 × [(1×23) + (1×35.5)] = 1.4625 g NaCl is required to dissolved in 250ml of water to obtain 0.1M NaCl. Wt. (gm) = 0.1 x 250 x 58.5 / 1000 = 1.4625 g NaCl is required to dissolved in 250ml of water to obtain 0.1M NaCl.
- Dissolve 9 g NaCl (mw 58.44) in 700 ml deionized or distilled water in clean container.
- Add water to bring total solution volume to 1000 ml.
- Make 10 ml aliquots in sterile 15 ml culture tubes.
So to make approximately 0.5N hydrochloric acid, you dilute the conc. HCl 24 times. To make a litre, you'd measure 42 ml of the conc. acid (because 1000/24=41.7) and add it to about 800 ml of water.
Dear Aspirant, To prepare 0.1N NaOH solution we should dissolve 40 grams of NaOH in 1L of water and to standardize we should Use titration method.
NaCl 11.7 g Distilled water to make 1 liter Dispense in suitable containers. Autoclave 15 min at 121°C.
1%NaCl sulution means hypertonic solution in which RBC cell shrinks.
A normal is one gram equivalent of a solute per liter of solution.
Define Normality and Its Formula
The equivalent value is calculated using the chemical species' molecular weight and valence. Normality is the primary concentration unit that the reaction activity can influence. When the normality of a solution is 1, it is referred to as a normal solution.
What does 1 normality mean?
A 1N solution contains 1 gram-equivalent weight of solute per liter of solution. Expressing gram-equivalent weight includes the consideration of the solute's valence.
A one percent solution is defined as 1 gram of solute per 100 milliliters final volume. For example, 1 gram of sodium chloride, brought to a final volume of 100 ml with distilled water, is a 1% NaCl solution.
This solution is used to supply water and salt (sodium chloride) to the body. Sodium chloride solution may also be mixed with other medications given by injection into a vein. This solution is usually given by injection into a vein as directed by your doctor.
0.8% salt solution is physiological saline and is thus, isotonic. In isotonic solutions, the cells remain the same as the osmotic pressure outside the RBC is same as the pressure inside the cells.
0.2% NaCl solution is hypotonic to RBCs so the RBCs become swollen due to endosmosis.
The molar mass of sodium chloride is 58.5 grams. The number of moles of sodium chloride= 0.085moles. The volume of the solution is considered as 100mL or 0.1L. Hence, the molarity of 5%saline solution is 0.85M.
3% and 5% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, hypertonic solution for fluid and electrolyte replenishment in single dose containers for intravenous administration. The pH may have been adjusted with hydrochloric acid. It contains no antimicrobial agents.
0.1% NaCl is hypotonic to red blood cells, and causes their lysis. This is due to NaCl entering the cell down its concentration gradient, followed by water.
Sodium Chloride 30% w/v Concentrate for Solution for Infusion should be diluted sufficiently to obtain an isotonic (0.9% w/v) solution. An isotonic solution can be prepared by diluting 30 mL Sodium Chloride 30% w/v Concentrate for Solution for Infusion to 1 litre with a non-electrolyte solution or water for injections.
Solutions containing NaCl concentrations including 0.18%, 0.2%, 0.3%, 0.45%, and 0.9% have been used for children with different clinical conditions. Solutions containing sodium concentrations close to the human serum sodium concentration of 135–145mmol/L are collectively called isotonic solutions.
What is the 0.1 N of NaCl?
0.1 normal solution of NaCl is found to be isotonic with 1.1 % solution of Urea.
0.1N NaOH or NaOH 0.1N means that the NaOH solution in 0.1 normal. The capital letter N is used to indicate the normality of a solution, i.e., it is the equivalent concentration. In normality, another factor, stoichiometry, is added to molarity. Normality is defined as molarity multiplied by a stoichiometric factor z.
Normal saline is 0.9% saline. This means that there is 0.9 G of salt (NaCl) per 100 ml of solution, or 9 G per liter. This solution has 154 mEq of Na per liter. In fact, all the other solutions listed on the previous screen will be compared to normal saline as if it has 150 mEq of Na/L.
The origin of normal saline has been traced to an 1883 study by a Dutch scientist named Hamburger. His work suggested, mistakenly, that the concentration of salts in human blood was 0.9 percent. He argued that a solution of equal concentration would be a "normal" composition for intravenous fluids, hence the name.
a) Remove and discard 50 ml from a 500ml bag of 0.45% Sodium Chloride b) To the remainder of the bag add 50 ml of 50% glucose. Add 7.5ml of concentrated 30% sodium chloride. Measure the sodium chloride very carefully.
Halides and alkaline metals dissociate and do not affect the H+ as the cation does not alter the H+ and the anion does not attract the H+ from water. This is why NaCl is a neutral salt.
A 0.5M solution of NaCl represents . 5 Moles or 29.22 grams (where one mole is 58.44g) of NaCl dissolved in 1 liter of water.
Molarity of NaCl =0. 2M.
...
BAM R63: Physiological Saline Solution 0.85% (Sterile)
| NaCl | 8.5 g |
|---|---|
| Distilled water | 1 liter |
For 0.9% Sodium Chloride Injection, USP, each 100 mL contains 900 mg sodium chloride in water for injection. Electrolytes per 1000 mL: sodium 154 mEq; chloride 154 mEq.
What is a 0.9% solution?
"Normal saline" is an aqueous solution of 0.9% NaCl. This means that normal saline can be prepared by measuring out 0.9 g of NaCl and diluting this amount of NaCl to a final volume of 100 ml's in water. This would be the same as diluting 9 g of NaCl to a final volume of 1 liter in water.
0.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride and water for injection. Each mL contains sodium chloride 9 mg. It contains no bacteriostat, antimicrobial agent or added buffer and is supplied only in single-dose containers to dilute or dissolve drugs for injection.
Therefore the normality of 0.5 m solution is 1.0N .
0.5% means 0.5 grams in 100 ml, so if you only need 50 ml, you need 0.5 g / 2 = 0.25 g agarose for a 50 ml gel solution.
- Dissolve about 14 g of iodine in a solution of 36 g of potassium iodide in 100 ml of water.
- Add three drops of hydrochloric acid and dilute with water to 1000 ml.
- Standardize the solution in the following manner.
Normal saline is 0.9% saline. This means that there is 0.9 G of salt (NaCl) per 100 ml of solution, or 9 G per liter. This solution has 154 mEq of Na per liter.
Description. Sodium chloride, 0.1N standardized solution is used for volumetric analysis in laboratories. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand.
0.9% NaCl SOLUTION IS ISOTONIC
The importance of isotonicity (effective osmolar concentration the same as that of plasma) as a parameter of crystalloid solutions was first identified by Hamburger,30 who demonstrated its importance in maintaining red blood cell volume.
Uppercase M is molarity, which is moles of solute per liter of solution (not solvent). A solution using this unit is termed a molar solution (e.g., 0.1 M NaCl is a 0.1 molar solution of sodium chloride).
One mol of NaCl (6.02 x1023 formulas) has a mass of 58.44 g.
What is .1M of NaCl?
A 1 molar (M) solution will contain 1.0 GMW of a substance dissolved in water to make 1 liter of final solution. Hence, a 1M solution of NaCl contains 58.44 g.
0.9% Sodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride and water for injection. Each mL contains sodium chloride 9 mg. It contains no bacteriostat, antimicrobial agent or added buffer and is supplied only in single-dose containers to dilute or dissolve drugs for injection.
Isotonic Solutions
An example of an isotonic IV solution is 0.9% Normal Saline (0.9% NaCl). Because the concentration of the IV fluid is similar to the blood, the fluid stays in the intravascular space and osmosis does not cause fluid movement between compartments.
1.99 g of NaOH must be diluted to 500 mL to prepare a 0.1N solution.