Formation of Alkynes Via Double Elimination Of Halides
- Alkynes can be produced from vicinal or geminal dihalides through double elimination reactions.
- The usual choice of base for these reactions is sodium amide (NaNH2)
- The first equivalent of strong base forms an alkenyl halide. The second equivalent forms the alkyne.
- If a terminal alkyne is formed, a third equivalent of base will be consumed, since the resulting alkyne proton is relatively acidic (pKa 25)
- Overall, this process can be used for the synthesis of alkynes from alkenes, through 1) halogenation of alkene 2) double elimination of dihalide
Table of Contents
- Elimination Reactions: Form C–C (pi), Break C–H And C–LG
- Elimination Of A Vinyl Halide To Give An Alkyne
- Formation of Alkynes From Double Elimination Of Vicinal Dihalides
- Formation Of Alkynes From Double Elimination Of Geminal Dihalides
- Why Is This Useful? Going From An Alkene To An Alkyne
- Notes
- (Advanced) References and Further Reading
1. Alkenes Via Elimination Reactions Of Alkyl Halides: Form C–C (pi), Break C–H And C–LG
We’ve gone through elimination reactions before – treatment of alkyl halides with base gives alkenes. Today we’ll discuss a pathway to get to alkynes from alkyl dibromides (which in turn can be made from alkenes) via double elimination.
Some time ago we discussed elimination reactions (See post: Elimination reactions) follow the general pattern below, where two adjacent bonds to carbon are broken – usually C-H and C-X, where X is a leaving group – and in place we form a new π bond.
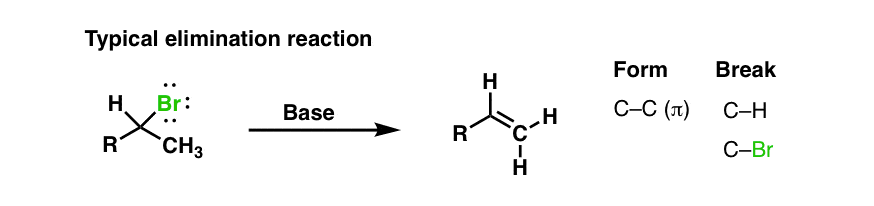
2. Alkynes From Alkenyl Halides: Elimination Of A Vinyl Halide To Give An Alkyne
If we can form alkenes through elimination reactions of alkyl halides, it’s natural to ask: can we also use elimination reactions to form alkynes? Perhaps through a reaction like this elimination of an alkenyl (aka vinyl) halide?

The answer is yes! Although to be fair, these types of molecules [alkenyl halides] are perhaps not the most familiar to us so far, We’ve only seen them once, and that was a post about how they can be synthesized from alkynes. (See post: Halogenation of Alkynes)
For our purposes, in order to synthesize them it’s common practice to start with an alkyl di halide.
In other words, we start with an alkyl di-halide, and then do two elimination reactions – one to form the alkenyl halide, and a second to form the alkyne.
Let’s have a look.
3. Alkynes From Double Elimination Of Vicinal Dihalides
There are two types of alkyl dihalides we’ve met so far. Vicinal dihalides have halogens on adjacent carbons – “in the vicinity”, if you will. Treatment of vicinal dihalides with strong base can lead to an elimination reaction [through the E2 mechanism] giving an alkenyl halide.
Treatment of this alkenyl halide with a second equivalent of base then gives the alkyne.
In previous posts we saw that a common base used for elimination reactions are alkoxide bases such as sodium ethoxide. But here, we typically go for a more powerful base, sodium amide (See post: Sodium Amide NaNH2)
Here’s an illustration of how it works.

In the first step, NaNH2 is the base in an elimination reaction [E2] to give the alkenyl bromide.In the second reaction, likewise a second equivalent of NaNH2 performs a second elimination reaction to form the alkyne.
This is one example – a rare example, I may add – of an elimination reaction that works on an sp2 hybridized carbon. You might recall seeing at some point that reactions of the SN1/SN2/E1/E2 types typically don’t occur on carbons other than sp3 hybridized systems. This is a rare exception!
Finally, what’s often not mentioned in this reaction is that the product alkyne, if terminal [i.e. has a C-H on the end] is acidic [pKa 25] – any excess NaNH2 will thus remove the alkyne C-H and give the alkynyl anion. So if a terminal alkyne is formed, three equivalents of NaNH2 will be consumed; the alkyne is protonated upon workup, usually by adding water.
4. Alkynes From Double Elimination Of Geminal Dihalides
Geminal dihalides contain two halogen atoms attached to the same carbon.
Treatment of geminal dihalides with NaNH2 likewise gives alkynes through two successive elimination reactions. [We haven’t really covered any reactions that form geminal dihalides, except for the di-addition of HX to alkynes. So if you happened to start off with an alkyne and made a geminal dihalide with it, this would be a way of getting the alkyne back].

In total, the elimination reactions described above represent a second way of making alkynes, other than through SN2 of acetylides with alkyl halides (See post: SN2 of Acetylides)
Let’s bring it back. What does it matter that we can do this?
OK. Let’s start putting some reactions together that show why this might be important.
5. Alkenes To Alkynes, Via Halogenation And Double Elimination
Let’s say we wanted to make an alkyne. But all we have to start with is an alkene. How might we get there?
- We’ve just learned how to make alkynes from vicinal dihalides.
- How might we make a vicinal dihalide from an alkene?
- Bromination!
So we can start off with an alkene… and brominate… and then add two equivalents of strong base to give ourselves the alkyne.

This is a quick example of multi-step organic synthesis. We will soon see much more of this in the context of alkynes!
Next Post: Alkynes Are A Blank Canvas
Notes
Related Articles
- Alkynes Are A Blank Canvas
- Synthesis (5) – Reactions of Alkynes
- Alkyne Halogenation: Bromination, Chlorination, and Iodination of Alkynes
- Reagent Friday: Sodium Amide (NaNH2)
- Acetylides from Alkynes, And Substitution Reactions of Acetylides
- The E2 Mechanism
- Alkyne Reactions Practice Problems With Answers (MOC Membership)
(Advanced) References and Further Reading
- Eliminations from Olefins
DR. G. KOBRICH.
Angew. Chem. Int. Ed. 1965 4 (1), 49
DOI: 10.1002/anie196500491
A review describing various types of a- and b-elimination reactions of alkenes to give alkynes. - Elimination Strategy for Aromatic Acetylenes
Orita, H.; Otera, J.
Chem. Rev. 2006, 106, 5387
DOI: 10.1021/cr050560m
Section 3.3 in this review covers the synthesis of alkynes by double dehydrobromination reactions from vic-dibromoalkanes. - Unsaturated eight-membered ring compounds. XI. Synthesis of sym-dibenzo-1,5-cyclooctadiene-3,7-diyne and sym-dibenzo-1,3,5-cyclooctatrien-7-yne, presumably planar conjugated eight-membered ring compounds
Henry N. C. Wong, Peter J. Garratt, and Franz Sondheimer
Journal of the American Chemical Society 1974 96 (17), 5604-5605
DOI: 10.1021/ja00824a066
The reaction used to synthesize the strained cyclooctyne is the double dehydrobromination reaction, using fairly standard conditions (KOtBu in THF). - Proton NMR study of two tetradehydrocyclodecabiphenylenes
Charles F. Wilcox Jr. and Karl A. Weber
The Journal of Organic Chemistry 1986 51 (7), 1088-1094
DOI: 10.1021/jo00357a028
The authors also use a double dehydrobromination reaction to obtain cyclic dialkynes, also using standard conditions (KOtBu in THF). The experimental section has detailed procedures.
In the realm of organic chemistry, the formation of alkynes through double elimination of halides is a fascinating and intricate process. The key players in this chemical ballet are vicinal and geminal dihalides, and the choreography involves precise steps of elimination reactions facilitated by a strong base, notably sodium amide (NaNH2).
The journey begins with vicinal dihalides, where halogens adorn adjacent carbons. When treated with sodium amide, a robust base, a double elimination process occurs via the E2 mechanism. The first equivalent of NaNH2 triggers the elimination, yielding an alkenyl halide. The second equivalent steps in, leading to the final act—the formation of the alkyne. What's intriguing is the use of sodium amide as the chosen base, a powerhouse that makes this reaction possible on an sp2 hybridized carbon—a rare exception in the organic chemistry repertoire.
The script doesn't end here; there's a geminal twist. Geminal dihalides, sporting two halogen atoms on the same carbon, also take center stage in alkynes' production. Once again, NaNH2 takes the lead, orchestrating two successive elimination reactions to bring forth the alkyne. This method represents a second avenue for generating alkynes, complementing other synthetic routes like SN2 of acetylides with alkyl halides.
But why bother with this intricate dance of molecules? The answer lies in the potential for synthesizing alkynes from alkenes. Imagine starting with a simple alkene and, through the magic of bromination and double elimination, transforming it into a complex alkyne. This multi-step organic synthesis opens up possibilities for crafting diverse molecular structures.
As we delve into this world of chemical transformations, the importance of these processes becomes evident. The ability to manipulate molecules at such a fundamental level provides a powerful tool for organic chemists, enabling them to design and create compounds with specific structures and properties.
So, whether you're navigating the intricacies of elimination reactions, pondering the role of sodium amide, or contemplating the artistry of organic synthesis, remember that the world of alkynes is a blank canvas waiting for the skilled hands of chemists to paint its vibrant molecular landscape.