- Last updated
- Save as PDF
- Page ID
- 881
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
The double bond of an alkene consists of a sigma (σ) bond and a pi (π) bond. Because the carbon-carbon π bond is relatively weak, it is quite reactive and can be easily broken and reagents can be added to carbon. Reagents are added through the formation of single bonds to carbon in an addition reaction.
.jpg?revision=1&size=bestfit&width=409&height=109)
Introduction
An example of an alkene addition reaction is a process called hydrogenation.In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane. Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product. In other words, the energy of the product is lower than the energy of the reactant; thus it is exothermic (heat is released). The heat released is called the heat of hydrogenation, which is an indicator of a molecule’s stability.


Although the hydrogenation of an alkene is a thermodynamically favorable reaction, it will not proceed without the addition of a catalyst.
Common catalysts used are insoluble metals such as palladium in the form Pd-C, platinum in the form PtO2, and nickel in the form Ra-Ni. With the presence of a metal catalyst, the H-H bond in H2 cleaves, and each hydrogen attaches to the metal catalyst surface, forming metal-hydrogen bonds. The metal catalyst also absorbs the alkene onto its surface. A hydrogen atom is then transferred to the alkene, forming a new C-H bond. A second hydrogen atom is transferred forming another C-H bond. At this point, two hydrogens have added to the carbons across the double bond. Because of the physical arrangement of the alkene and the hydrogens on a flat metal catalyst surface, the two hydrogens must add to the same face of the double bond, displaying syn addition.
Common Applications
Hydrogenation reactions are extensively used to create commercial goods.Hydrogenation is used in the food industry to make a large variety of manufactured goods, like spreads and shortenings, from liquid oils. This process also increases the chemical stability of products and yields semi-solid products like margarine. Hydrogenation is also used in coal processing. Solid coal is converted to a liquid through the addition of hydrogen. Liquefying coal makes it available to be used as fuel.
References
- "Catalysts that improve coal hydrogenation." Chemical Week 132 (1983): 38.
- List, G., M. Jackson, F. Eller, and R. Adlof. “Low trans spread and shortening oils via hydrogenation of soybean oil.” Journal of the Americal Oil Chemists 84 (2007): 609-612.
- Singh, D., M. Rezac, and P. Pfromm. “Partial Hydrogenation of Soybean Oil with Minimal Trans Fat Production Using a Pt-Decorated Polymeric Membrane Reactor.” Journal of the American Oil Chemists Society 86 (2009): 93-101.
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry: Structure and Function. New York: W.H. Freeman and Company, 2007.
- Zumdahl, Steven S. Chemistry. Lexington, Massachusetts: D.C. Heath and Company, 1993.
Problems
Complete the following reactions. Provide stereochemistry if necessary.
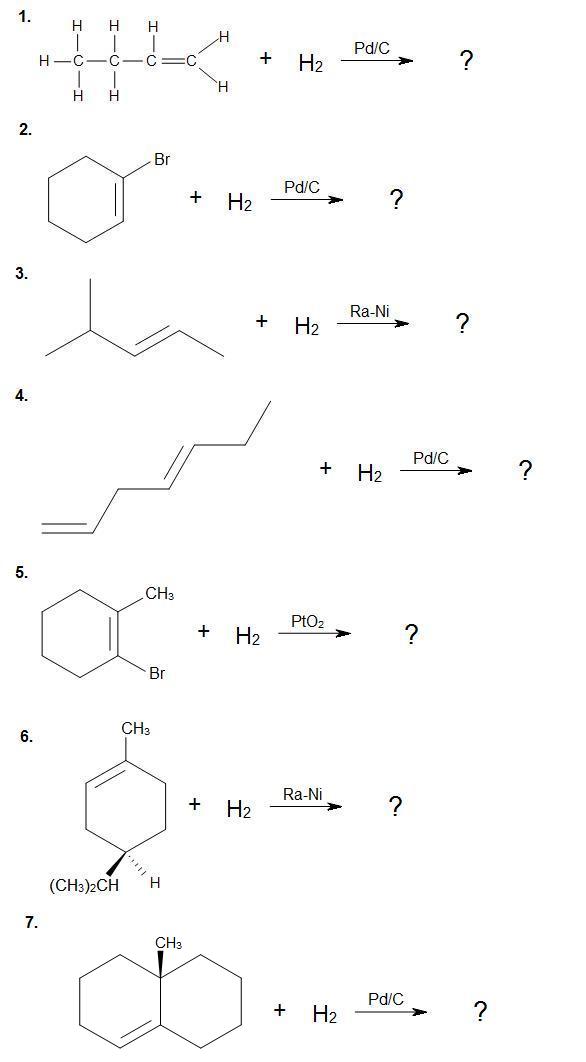.jpg?revision=1&size=bestfit&width=520&height=939)
Contributors
- Jennifer Lew (UCD)
I am a chemistry enthusiast with a deep understanding of organic chemistry, particularly in the realm of alkene reactions. My expertise extends to the mechanisms and applications of various chemical processes. To demonstrate my knowledge, let's delve into the concepts discussed in the provided article.
The article discusses the chemistry of alkene addition reactions, focusing on hydrogenation as an example. The double bond of an alkene comprises a sigma (σ) bond and a pi (π) bond. The π bond is relatively weak, making it reactive and susceptible to addition reactions.
In a hydrogenation reaction, two hydrogen atoms are added across the double bond, resulting in a saturated alkane. This process is thermodynamically favorable, leading to the formation of a more stable product with lower energy. The energy released during this exothermic reaction is known as the heat of hydrogenation, serving as an indicator of the molecule's stability.
The article emphasizes the need for a catalyst in hydrogenation reactions. Common catalysts include insoluble metals like palladium (Pd-C), platinum (PtO2), and nickel (Ra-Ni). The metal catalyst facilitates the cleavage of the H-H bond in H2, and hydrogen atoms are transferred to the alkene, forming new C-H bonds. Notably, the physical arrangement of the alkene and hydrogens on a flat metal catalyst surface results in syn addition, where two hydrogens add to the same face of the double bond.
Hydrogenation reactions find extensive applications in various industries. In the food industry, they are used to produce spreads and shortenings from liquid oils, enhancing product stability. Additionally, hydrogenation is employed in coal processing, converting solid coal into a liquid form for use as fuel.
To further support these concepts, references to reputable sources such as "Organic Chemistry: Structure and Function" by K. Peter C. Vollhardt and Neil E. Schore, and "Chemistry" by Steven S. Zumdahl are provided.
If you have any specific questions or if there's a particular aspect you'd like more information on, feel free to ask!


.jpg?revision=1&size=bestfit&width=720&height=760)